Longer Term All-Cause and Cardiovascular Mortality With Intensive Blood Pressure Control
A Secondary Analysis of a Randomized Clinical Trial
Wake Forest University School of Medicine
Disclosures
I have nothing to disclose.
TL;DR
After SPRINT, from 2016-2020, the mean systolic blood pressure levels of participants returned to about 140 mm Hg, and the benefit of intensive blood pressure control attenuated.
Additional follow-up from 2020-2023 provides an opportunity to think carefully about interventions that had a beneficial effect.
Slides are available at https://www.byronjaeger.com/talk/ (google byron jaeger biostats talks)
Overview
Background: SPRINT
Why is longer term follow-up relevant?
How did SPRINT do longer term follow-up on all-cause and CVD mortality?
Results
Conclusion
SPRINT
Two treatments with different systolic blood pressure (SBP) targets:
- Standard: SBP target of < 140 mm Hg
- Intensive: SBP target of < 120 mm Hg
Participants:
- aged 50 years or older
- with hypertension and increased cardiovascular risk
- without diabetes or history of stroke
Why do longer term follow-up?
Investigate the legacy effect of intensive treatment
Does targeting SBP < 120 mm Hg have long term benefits or harms compared to targeting SBP < 140 mm Hg?
Longer term all-cause/CVD mortality
We assessed all-cause1 and cardiovascular disease2 (CVD) mortality post-trial via the US National Death Index (NDI) from 2016-2020 (Jaeger et al. 2022).
Among 2944 trial participants,3 post-trial SBP levels were analyzed (Drawz et al. 2020).
Results
Number of events
2016-2020
- 1644 all-cause mortality events
- 521 CVD mortality events
2016-2023
- 2597 all-cause mortality events
- 907 CVD mortality events
Difference:
- 953 additional all-cause mortality events
- 386 additional CVD mortality events
- No additional analyses on BP
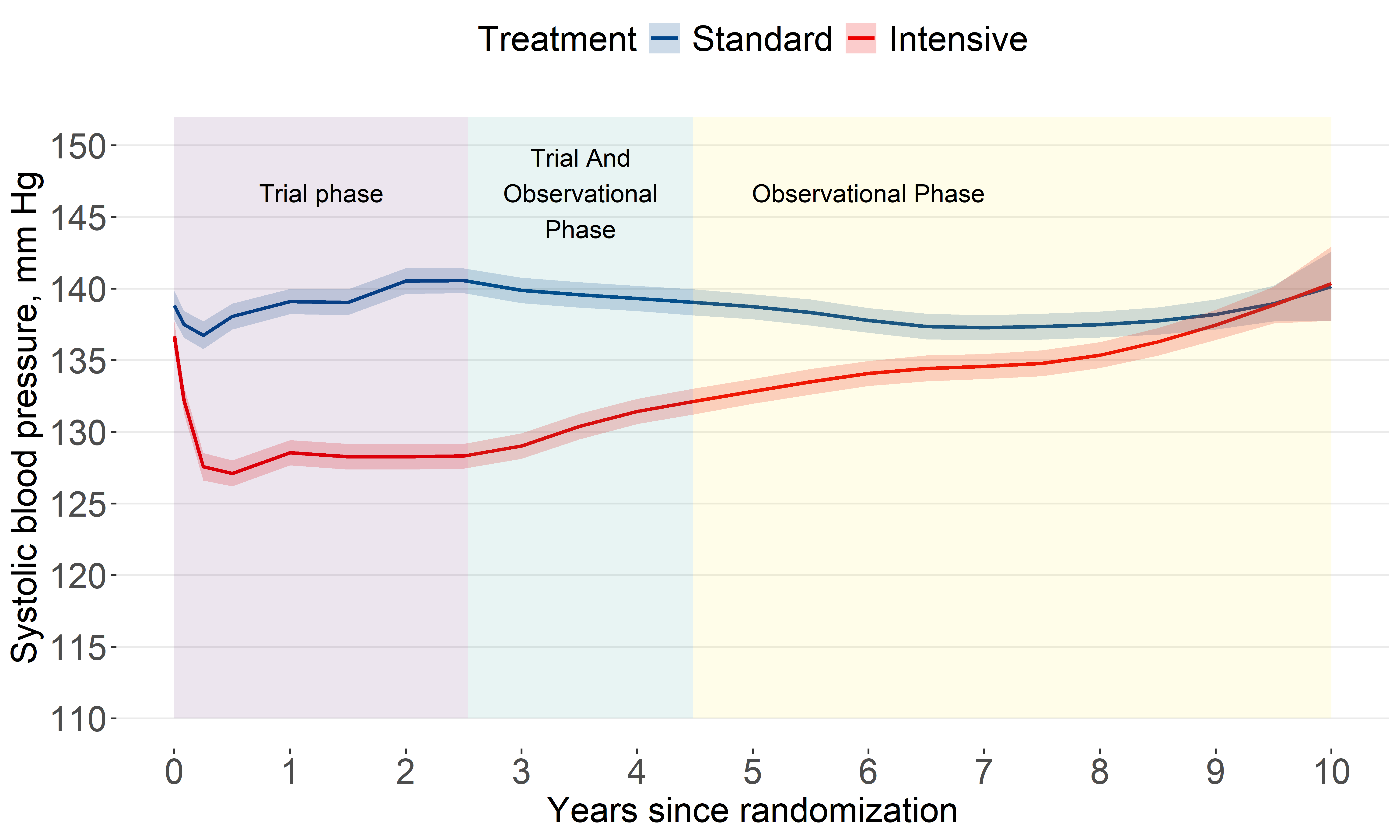
Systolic BP levels
SBP levels among the intensive group increased after the trial.
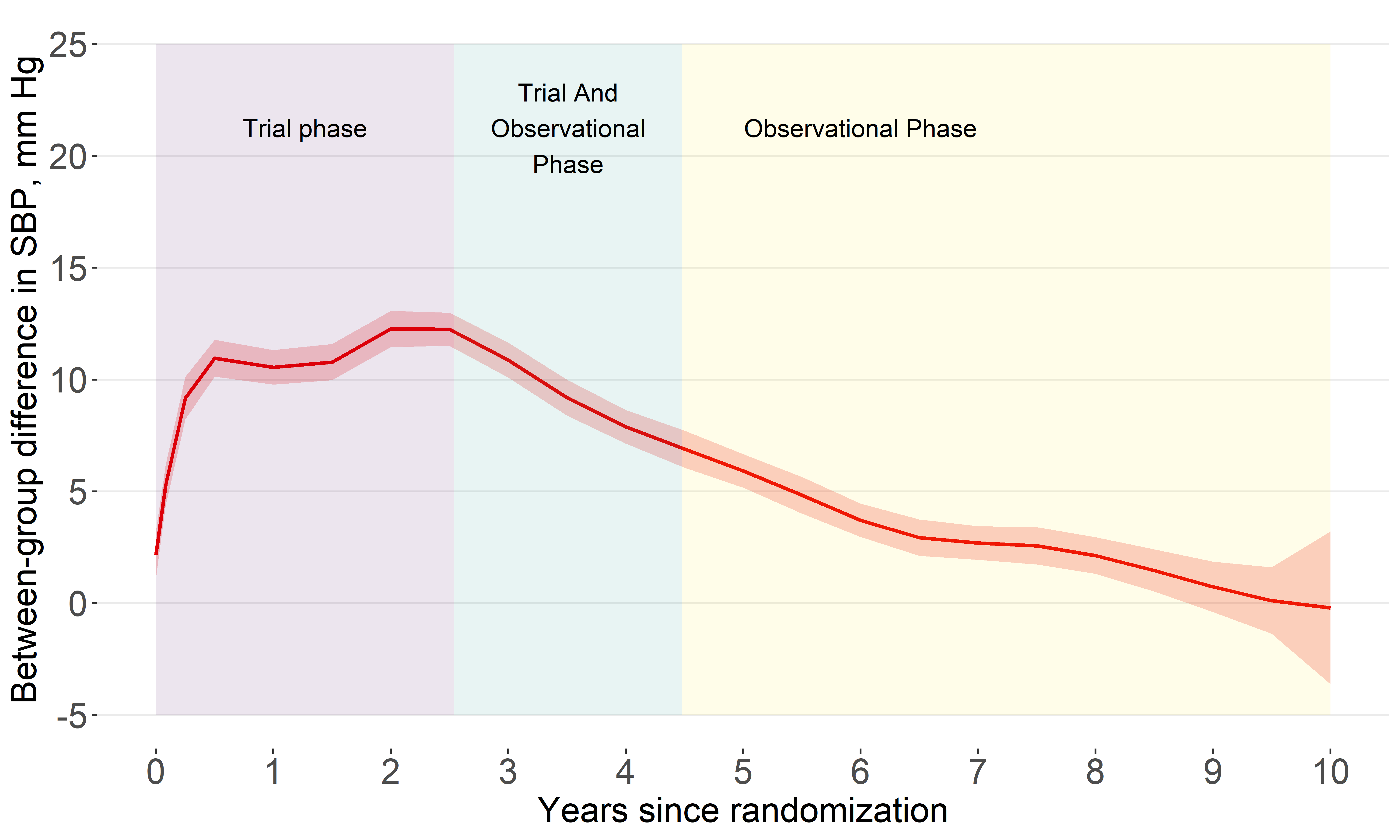
Systolic BP difference
By year 10, the estimated difference was about 0
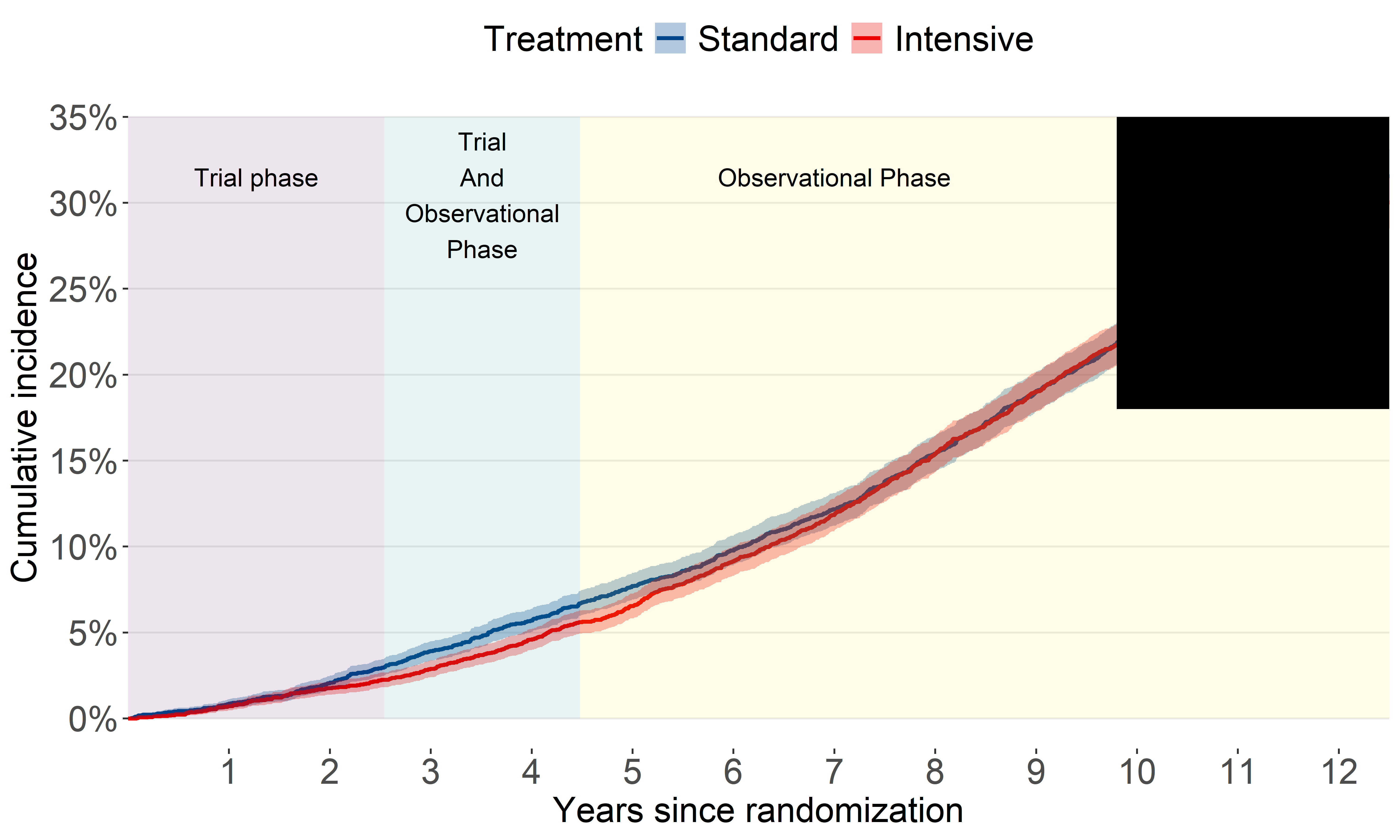
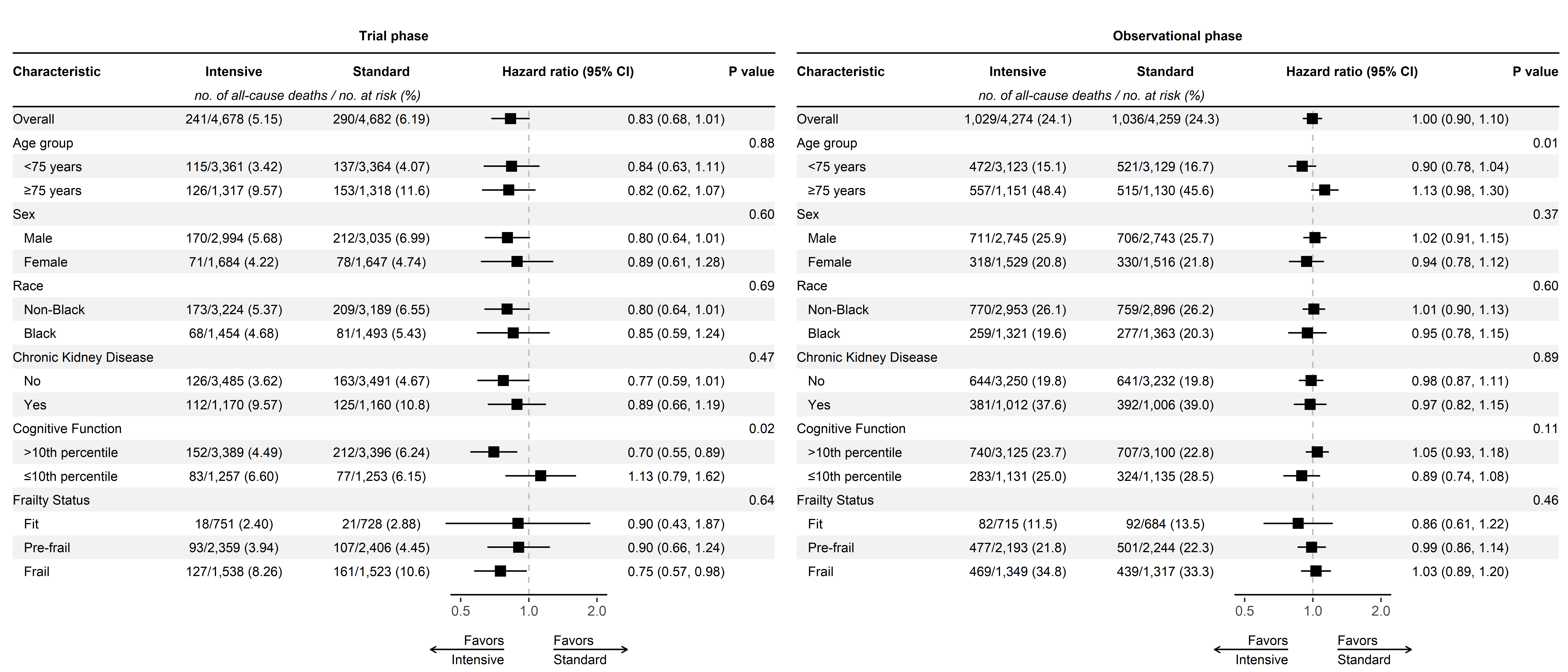
All-cause mortality (2016-2020)
Our original analysis showed the benefit for all-cause mortality attenuated quickly
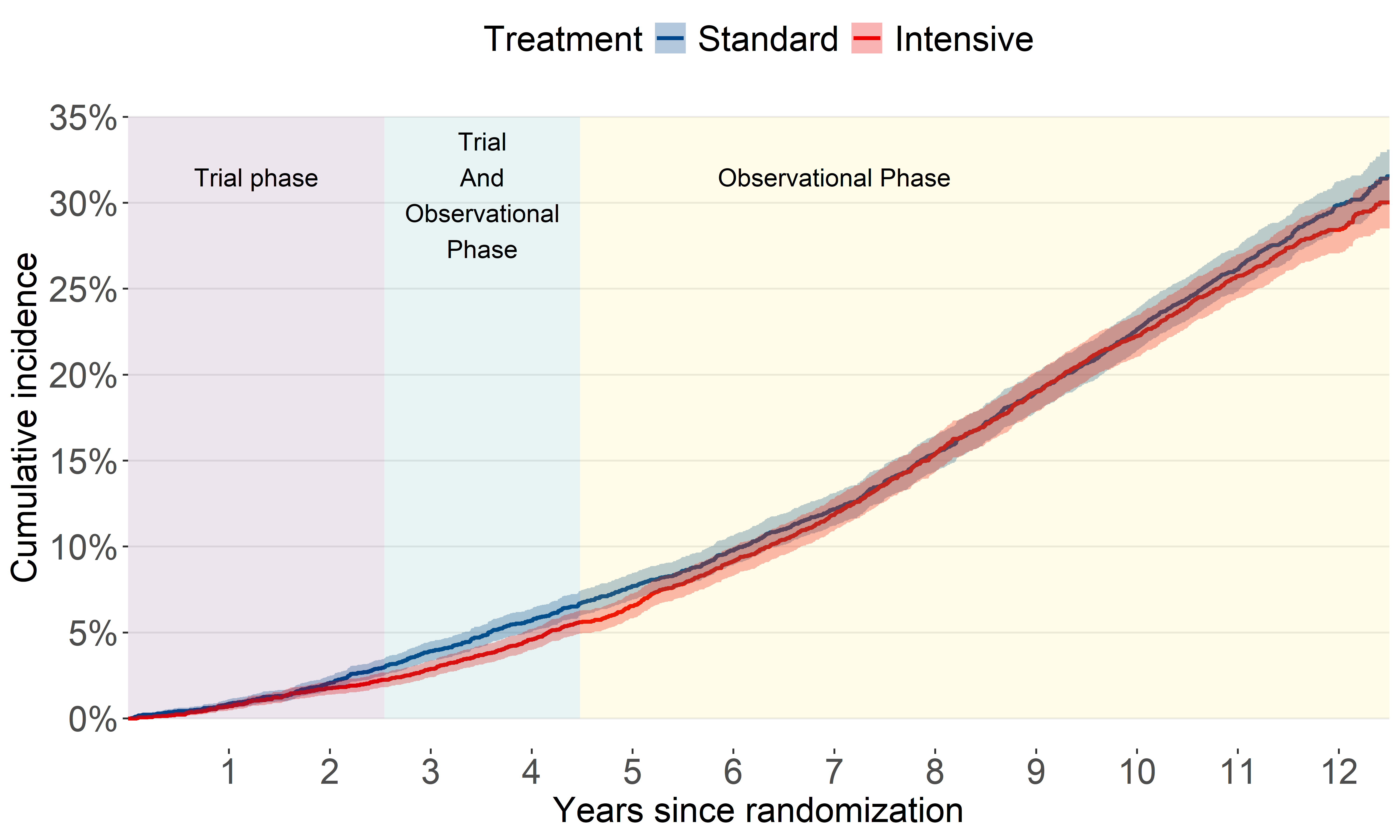
All-cause mortality (2016-2023)
This update shows an unexpected separation after several years of overlap
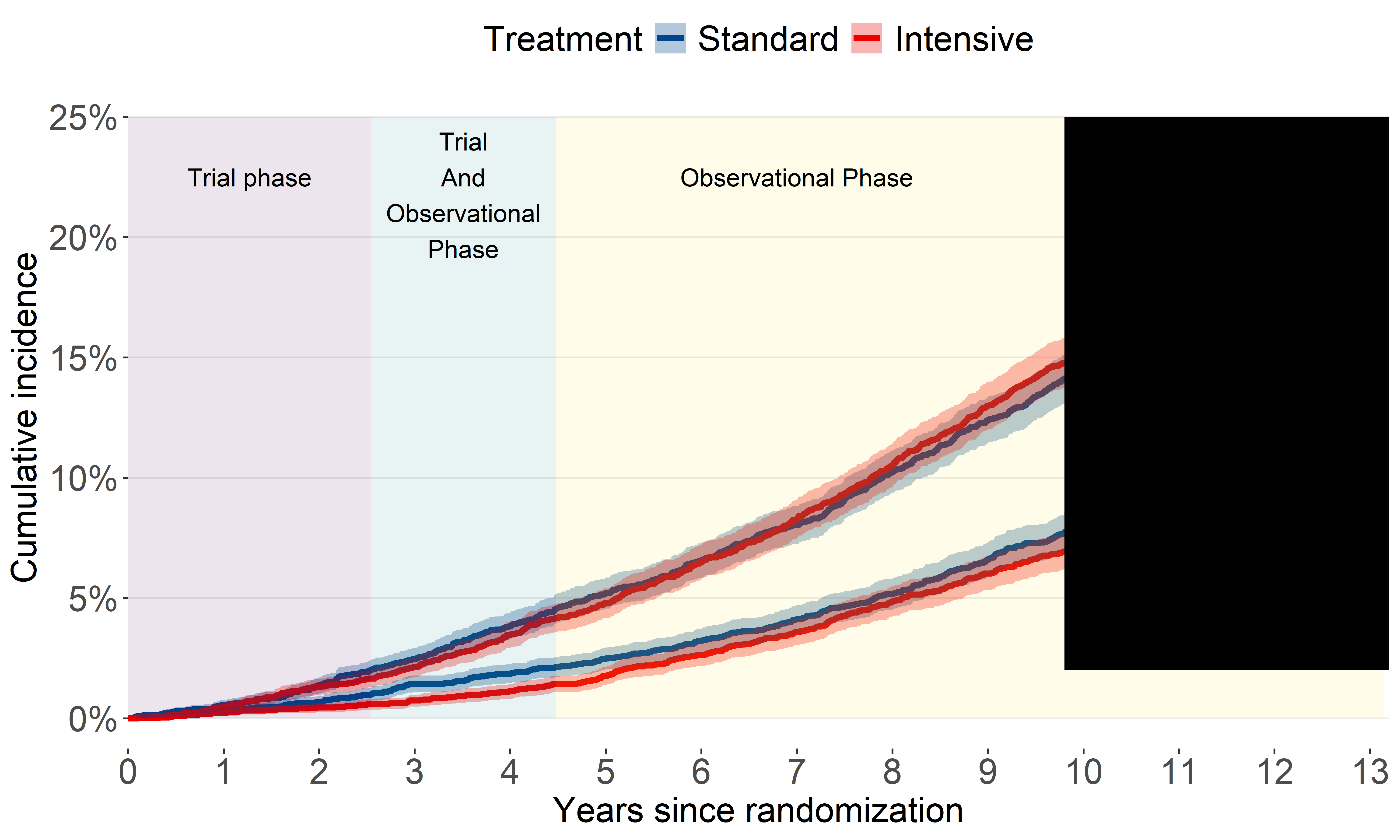
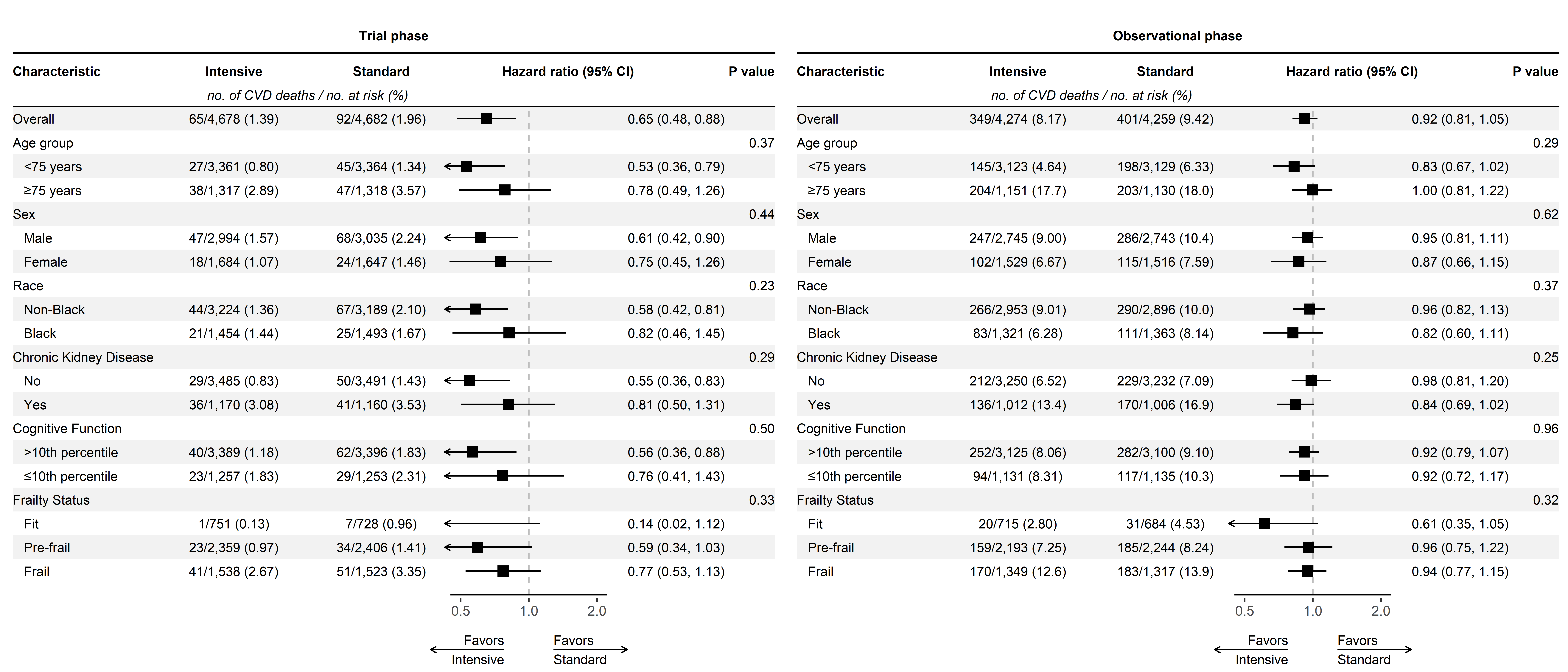
CVD mortality (2016-2020)
Our original analysis showed benefit for CVD mortality attenuated.
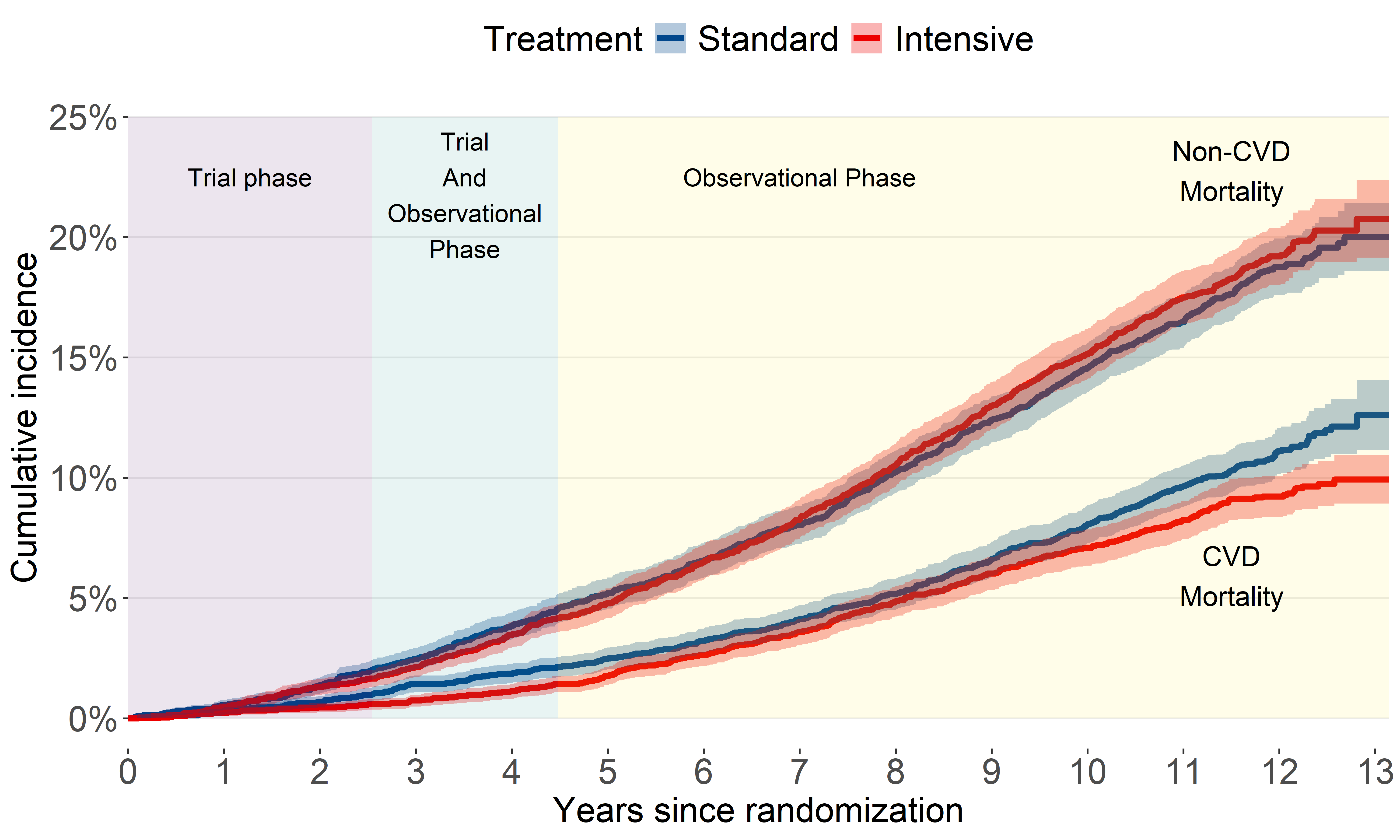
CVD mortality (2016-2023)
A bit of a head-scratcher.
Conclusion
After SPRINT, from 2016-2020, SBP returned to about 140 mm Hg, and the benefit of intensive blood pressure control attenuated.
The latest update shows what appears to be a resurfaced benefit in terms of CVD mortality. This could be attributed to:
participants in the intensive blood pressure control group who maintained control after the trial
intensive blood pressure control has higher estimated benefit for younger adults.
References
BONUS ROUND
How did we analyze longer term follow-up?

assumed treatment group differences would not be constant over time.
split each participant’s follow-up time into non-overlapping trial and observational phases
estimated regression coefficients for intensive treatment separately during each phase.
All-cause mortalty (time-split analysis)
CVD mortalty (time-split analysis)
Footnotes
Deaths were treated as confirmed if they were a Class 1 match, or a Class 2, 3, or 4 match with a probabilistic score above cutoffs recommended by the NDI.
Cardiovascular mortality for NDI-based follow-up used the NDI Plus System, which automatically identifies underlying causes of death from death certificates, including conversion to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes. We defined CVD mortality as any death containing the ICD-10 codes of I00 to I99
We identified 3074 participants with 3 or more electronic health record reports of outpatient blood pressure measurements during the trial. After excluding 130 participants without electronic health record data following July 2016 (ie, conclusion of the trial), a total of 2944 patients were included for the ancillary blood pressure analysis.